Aluminum methylamidoborane complexes: mechanochemical synthesis, structure, stability and reactive hydride composites
T. Zhang, M. Devillers*, Y. Filinchuk*
Institute of Condensed Matter and Nanosciences (IMCN), Université catholique de Louvain, Belgium
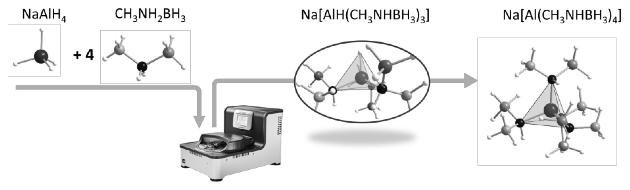
Over the past years, hydrogen has been identified as the most promising clean energy carrier. One of the main obstacles to developing wide hydrogen applications is lacking safe, compact, cost-efficient hydrogen storage methods. Compared with methods of physical storage, materials-based hydrogen storage has been considered a long-term solution[1]. Recently, Aluminium-amidoborane complexes[2-5] have attracted much attention due to their promising hydrogen storage properties. However, the molecular-level understanding of the relationships between the structure and formation or properties is quite insufficient due to the few reported examples and minimal studies. Here we studied the synthesis, structure, and hydrogen storage properties of the Al-based terminal substituted amidoborane complexes Na[AlH(CH3NHBH3)3] and Na[Al(CH3NHBH3)4] obtained from NaAlH4 and CH3NH2BH3. Both crystallize in a monoclinic unit cell with space group symmetry P21/n. Compared with unsubstituted Al-based amidoboranes (Na[Al(NH2BH3)4]), Na[Al(CH3NHBH3)4] is easier to obtain with less energy input. However, large mass losses during the thermal decomposition of Na[Al(CH3NHBH3)4], exceeding largerly the hydrogen content of this complex hydride, indicates the loss of larger fragments, preventing the reversibility of H-desorption. Fortunately, Na[Al(CH3NHBH3)4] + 12 NaH and Na[Al(CH3NHBH3)4] + 6 NaNH2 reactive hydride composites (RHCs) decreased the mass loss significantly. Especially, the latter RHC shows desorption of 6.77 wt% of high purity hydrogen at moderate temperatures. This gives hopes for the potential hydrogen reversibility in this and related Al-amidoborane systems.
- Ren, J.; Musyoka, N. M.; Langmi, H. W.; Mathe, M.; Liao, S., International Journal of Hydrogen Energy 2017, 42, 289-311.
- Xia, G.; Tan, Y.; Chen, X.; Guo, Z.; Liu, H.; Yu, X., Journal of Materials Chemistry A 2013, 1, 1810-1820.
- Dovgaliuk, I.; Jepsen, L. H.; Safin, D. A.; Łodziana, Z.; Dyadkin, V.; Jensen, T. R.; Devillers, M.; Filinchuk, Y., Chemistry – A European Journal 2015, 21, 14562-14570.
- Yang, J.; Beaumont, P. R.; Humphries, T. D.; Jensen, C. M.; Li, X., Energies 2015, 8.
- Møller, K. T.; Jørgensen, M.; Andreasen, J. G.; Skibsted, J.; Łodziana, Z.; Filinchuk, Y.; Jensen, T. R., International Journal of Hydrogen Energy 2018, 43, 311-321.
