-
Share this page
P16-Guillaume
Growth Mechanisms of CVD Diamond
E. Guillaume1,2,3, D. E. P. Vanpoucke1,2, L. Henrard3 and K. Haenen1,2
1Instituut voor Materiaalonderzoek (IMO), Universiteit Hasselt
2IMOMEC, IMEC vzw
3Laboratoire de Physique du Solide, Département de Physique, Université de Namur
Atomic-scale mechanism involved at chemical vapour deposition condition during diamond growth are of great interest for both experimental and theoretical diamond researchers. Early studies revealed multi-steps pathways to grow diamond from a flat H-terminated (100) diamond surface, by means of Quantum Mechanics/ Molecular Mechanics. However, a recent study showed that some part of the former model [1,2] were unable to bear the weight of further investigations, as some of the earliest results were biased by the hybrid QM/MM methods [1]. This work uses full quantum mechanical modelling (using the VASP implementation of Density Functional Theory) to perform spin-polarised climbing Nudged Elastic Band [3] calculations to unravel the properties of the transition state of successive reactions. The resulting very accurate energy barriers provide indication on the nucleation of additional layers to a diamond surface.
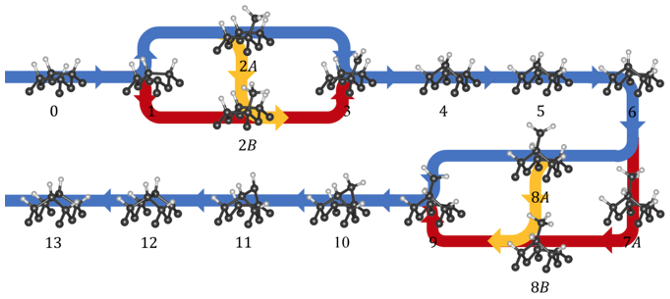
Additionally, we assess earlier suggestions about the migration likelihood of component such as methyl, hydrogen or vacancy on (100)-oriented surfaces.
We also assess the fact that both spin-polarised calculation and van der Waals corrections must be considered as some reaction happening on the surface turns out to be strongly spin-dependent, and that the energy barriers accounting for van der Waals interaction leads the way to better agreement with experimental observations. Finally, our atomic-scale results provide interesting dataset for multi-scale approaches in order to better understand the growth of CVD grown diamond.
References:
1. A. Cheesman, J. N. Harvey, and M. N. R. Ashfold, The Journal of Physical Chemistry A, vol. 112 (2008), pp. 11436–11448
2. L. M. Oberg, M. Batzer, A. Stacey, and M. W. Doherty, Carbon, vol. 178 (2021), pp. 606–615
3. G. Henkelman, B. P. Uberuaga, and H. Jónsson, The Journal of chemical physics, vol. 113 (2000), pp. 9901–9904
