-
Share this page
P23-Maertens
Novel Zr-bases heterogeneous catalysts for the synthesis of γ-valerolactone: a focus on the main parameters influencing the activity
Amélie Maertens,# Loraine Soumoy,# Carmela Aprile*
Laboratory of Applied Materials Chemistry, Unit of Nanomaterials Chemistry, NISM,
University of Namur, Rue de Bruxelles, 61. 5000 Namur
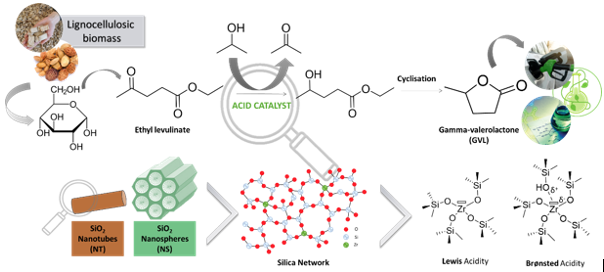
With the rising concerns about environmental pollution and the short-term fossil fuel depletion, the development of sustainable alternatives to petroleum-based products recently gained attractiveness. The use of renewable feedstocks such as biomass derivatives to produce new green chemical platforms is among the most promising approach.1 In particular, the catalytic transformation of lignocellulosic biomass (i.e. nonfood biomass) into γ-valerolactone (GVL) recently drawn considerable attention. The latter chemical can be used as liquid fuel or fuel additive, green solvent and constitutes a versatile intermediate for various chemical reactions.2
GVL is obtained from the hydrogenation of levulinic acid (LA). This reaction is commonly performed employing an elevate hydrogen pressure in the presence of a noble metal catalyst. A greener and low-cost process would be to use non-noble metal catalyst and alcohol as hydrogen donors through a Meerwein-Pondorf-Verley (MPV) reaction. To catalyze MPV reaction, materials with a combination of Lewis and Brønsted acidic centered (e.g. Sn, Zr as single site within a silica matrix) are known to be notably efficient.3 To support these catalytic active species, mesoporous silicates are particularly valuable.
In this work, we developed novel mesoporous silica with nanostructured morphology embedding zirconium species. SiO2 nanotubes and nanospheres are highly innovative structures. The impact of both the nature of the active sites and the morphology of the materials were investigated. All the materials were characterized in depth and tested as catalysts for the synthesis of GVL. The best solids displayed excellent activity for the valorization of levulinic acid into γ-valerolactone and better performances than other previously reported materials.
References:
1. Corma, A., Iborra, S., Velty, A., Chemical Reviews, 2007, 107, 2411-2502.
2. Dutta, S., Yu, I. K. M., Tsang, D. C. W., Ng, Y. H., Ok, Y. S., Sherwood, J., Clark, J. H., Chemical Engineering Journal, 2019, 372, 992-1006.
3. He, J., Li, H., Xu, Y., Yang, S., Renewable Energy, 2020, 146, 359-370.
#These two authors contributed equally to this work, *carmela.aprile@unamur.be
